


15 + 111 points
Document Dissolution by rongo rongo
April 14th, 2007 2:29 PM
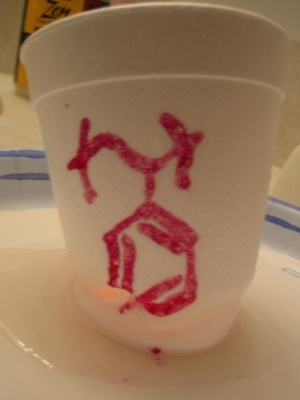
I used to be a chemist, but these days I rarely dissolve, precipitate, or distill. So I was excited to have this excuse to do some dissolving, but I wanted to turn the task inside out. I selected polystyrene (styrofoam cup) as the thing to dissolve, and put some ethyl acetate (acetone free nail polish remover) into the cup. Although I did not use enough solvent to dissolve the entire cup, I did dissolve the bottom part of it. Also, I wrote the chemical structure of polystyrene onto the cup using nail polish, which also contains ethyl acetate, so the ink dissolved some of the cup too.
cup1.jpg

You can see the cup starting to cave in at the bottom, and also where the ink has dissolved some of the surface.
23 vote(s)
5























Ohrlyeh Totenkinder
5
Jackie H
5
Sean Mahan
5
Ziggy C.
5
Murdoc
5
K!
5
d@n s
5
Blue
5Laura
5
Caroline The Curly
5
Mike Hellstrom
5
teucer
5
Lincøln
5
Coreopsis Major Bloden Melen
5
Spidere
5
GYØ Ben
5
Darkaardvark
5
Vee
5
LittleMonk
5
Garret Sollinger
5
Juliette
3
Edwin Farnham Butler III
3
Not Here No More
Terms
(none yet)2 comment(s)
posted by Coreopsis Major Bloden Melen on January 29th, 2008 8:08 AM
I've made polyvinyl chloride in the lab before, but I've never seen polystyrene melted! Nicely done!





















omg, thats amazingly awesome!! I love chemistry, and just had to see your praxis the moment I saw the picture of the cup with polystyrene's structure on it. way way cool. totally beyond expectations of awesomeness!!